Why buy from us?
Lorem Ipsum is simply dummy text of the printing and typesetting industry.
Ask a Question About This Product
- Stock: In Stock
- Brand: OEM
- Model: DSAK1002
- Location: https://detail.1688.com/offer/639541459850.html
Available Options
Data download link:https://pan.baidu.com/s/18Gl7cX-ONomwtdq2gmeBLw[Unable to copy link on mobile phone, please contact online customer service to send link]
PH range: 0-14PH
What is the difference between rechargeable and non rechargeable pH probes?
rechargeableThe pH composite electrode has a liquid addition hole on the electrode shell. When the external reference solution of the electrode is lost, the liquid addition hole can be opened to replenish KCl solution. The non rechargeable pH composite electrode is filled with gel like KCl, which is not easy to drain and has no feeding hole.
rechargeableThe characteristics of pH composite electrode are that the reference solution has a high permeation rate, the liquid interface potential is stable and reproducible, and the measurement accuracy is high. And when the reference electrode is reduced or contaminated, KCl solution can be replenished or replaced, but the disadvantage is that it is more difficult to use. When using rechargeable pH composite electrodes, the filling hole should be opened to increase liquid pressure and accelerate electrode response. When the dielectric liquid level is 2cm below the filling hole, new dielectric liquid should be replenished in a timely manner.
Non rechargeableThe characteristic of pH composite electrode is simple maintenance and convenient use, therefore it has been widely used. However, when used as a laboratory pH electrode, under long-term and continuous usage conditions, the concentration of KCl at the liquid interface will decrease, affecting the accuracy of the test. Therefore, when non rechargeable pH composite electrodes are not in use, they should be soaked in electrode soaking solution, so that the electrode performance will be good in the next test. However, most laboratory pH electrodes are not tested continuously for a long time, so this structure has a relatively small impact on accuracy.
Product Introduction
Working voltage: 5 ± 0.2V (AC/DC)
Working current: 5-10mA
Detectable concentration range: PH0-14
Temperature range for detection: 0-80 ℃
Response time: ≤ 5 seconds
Settlement time: ≤ 60 seconds
Component power: ≤ 0.5W
Working temperature: -10~50 ℃ (nominal temperature 20 ℃)
Humidity: 95% relative humidity (nominal humidity 65% relative humidity)
PH testing module size: 42mm * 32mm * 20mm
Output: Analog voltage signal output
Equipped with 4pc M3 mounting holes
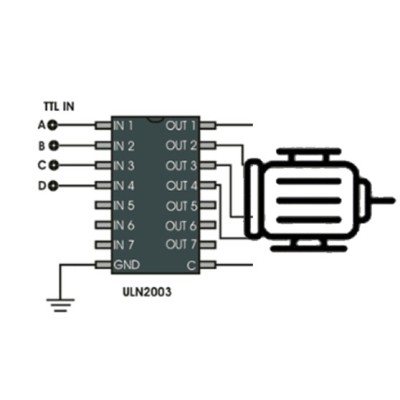
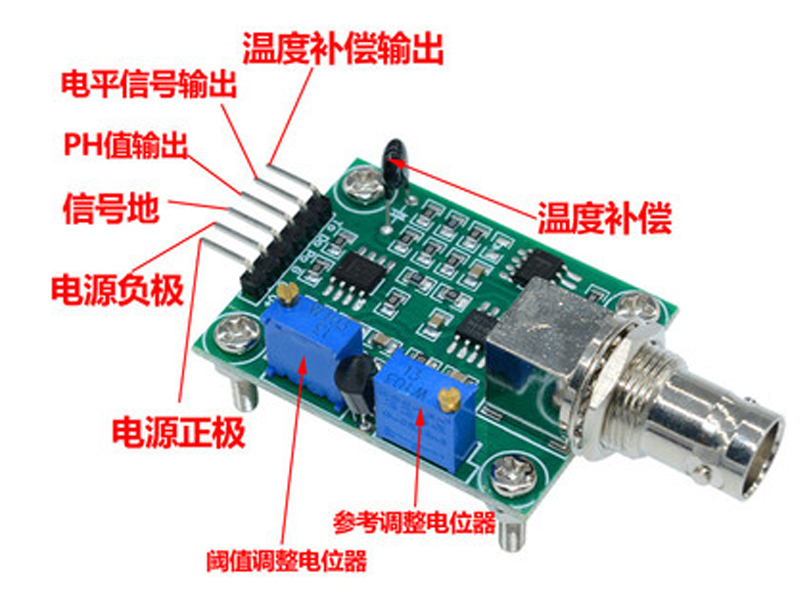
Module interface description:
Module manual/schematic/testing program,Contact online customer service after purchase to send
Use, maintenance, and upkeep of pH electrode probes:
1: Preparation before use
oneGently remove the protective bottle from the top of the electrode and store it for future use.
2. Clean the white deposited salt on the outside of the electrode with deionized water.
3. In order to maintain an appropriate permeation rate, the liquid level inside the tube must be below the end of the spiral glass tube and at least 1 inch (2.5cm) above the sample liquid level. When using, place the electrode bulb end completely in the measured liquid, allowing the bulb to fully contact the measured liquid and produce an ion reaction effect.
4. Gently shake the electrode (just like shaking a thermometer) to remove any air bubbles inside the electrode.
5. Soak the electrode in the pH electrode storage solution for 1 hour. If there is no electrode storage solution, 1g KCl can be added to 200mL of pH 7 buffer solution as a temporary electrode storage solution. The electrode can be configured with a long-term storage solution to activate and protect the bulb end when not in use. The preparation method involves adding 10mL of 3.3kcl solution to 200mL of pH 4 buffer solution. [3.3KCL is saturated potassium chloride, the preparation method is to weigh 23 grams of potassium chloride and mix it with 100ml of deionized water] An appropriate amount of preservative
2: Precautions for using electrode probes
oneUse fresh buffer solution
twoOpen the electrode protective cover
threeMeasure the gap and rinse the electrode with deionized water
fourStir buffer and sample: a) Stir at the same rate continuously; b) Stir first, then measure
fiveWhen preparing the pH electrode, rinse the electrode with distilled water before and after measurement. Use a lint free cloth to remove excess water from the electrode tip, avoiding friction with the electrode bubbles, as this can generate static electricity and interfere with accurate pH measurement.
sixEnsure that the buffer solution is at the same temperature as the sample. If the sample temperature is different, please use a temperature compensation probe for temperature compensation.
sevenRegularly check the electrode slope using two-point calibration method. When the electrode reading drifts or the slope is below 92%, please refer to the electrode cleaning instructions.
Three:Maintenance of electrode probes
oneCleaning of electrodes
Universal cleaning solution - Soak the electrode in 0.1M HCl or 0.1M HNO3 solution for 15 minutes, and then soak the electrode in electrode storage solution for 30 minutes.
twoLiquid interface blockage (salt precipitation)
Causes: hard water, mud, organic matter and dirt, floating microorganisms in water, moss, etc. Attach around the bubble and PTFEThe seepage on the sand core caused the external reference electrode to malfunction.
resolvent:Soak the electrode in hot water (60 ℃) for 15-20 minutes; Soak the electrode in a hot saturated KCl (60 ℃) solution for 20-30 minutes and cool to room temperature; Soak the electrode in pH 4 buffer solution for 20 to 30 minutes.
Check for excessive crystallization. If so, rinse the crystals repeatedly with deionized water and check if the flow rate is normal
If not available, soak the electrode in hot water (60 ℃) for 15-20 minutes; Soak the electrode in a hot saturated KCl (60 ℃) solution for 20-30 minutes and cool to room temperature; Soak the electrode in pH 4 buffer solution for 30 minutes. Soak the electrode tip in concentrated HCl for 5-10 minutes, rinse the electrode, and check if the electrolyte flow rate is normal. If the liquid interface is still blocked, pull the liquid interface (do not touch the glass bulb)
4:Precipitation of Inorganic Substances on pH Sensitive Membrane
Cause: Measurement of inorganic samples
Solution: Clean with EDTA, ammonia or acid
5:Dehydration of pH sensitive membrane
Causes: Improper storage, prolonged use, high temperature operation or strong alkaline solution, resulting in slow and unstable response
Solution: Activate the electrode
Soak the electrode in 0.1 M HCl for 1 minute, rinse with tap water for 30 seconds, soak the electrode in 0.1 M KOH for 1 minute, rinse with tap water for 30 seconds, calibrate the test electrode with buffer solution. If it still doesn't work, repeat the above steps up to 3 times
6:PHLong term use of electrodes without proper cleaning and maintenance in the tested environment can lead to unstable electrode data and other factors. Solution: After 2-3 months of electrode use, perform a bubble cleaning on the electrode and inspect the liquid contact area. Solution: Remove the electrode from the usage environment and check if there is any dirt on the surface of the bulb. If there is, gently wipe it with ethanol cotton and then clean it with deionized water.
Note that the bubbles are fragile, and attention should be paid during the wiping process. Then check the liquid contact area to see if there are any pollutants adhering to its surface. If there are, rinse with deionized water or shake the electrode back and forth in the measuring cup. If the water quality is not clean, change the water until the dirt disappears. Then clean the electrode and place it in the activation solution for 8 hours. Measure it in the standard solution to restore its measurement state.
Regarding the issue of receiving transaction records:
It is normal for the liquid to flow out of the front-end tube of the product, which is crystalline and does not affect its use. The protective sleeve of the front terminal contains liquid to protect the glass bulb in front. As long as the liquid inside the rod does not flow out, it does not affect its use